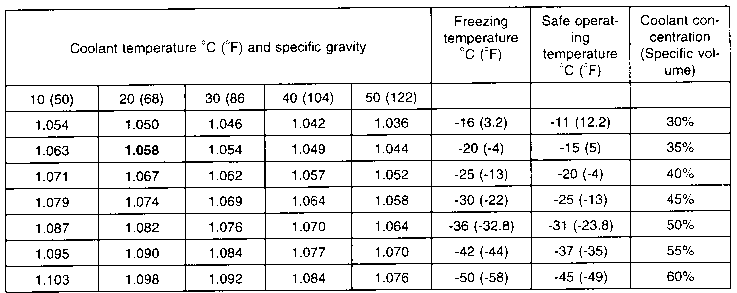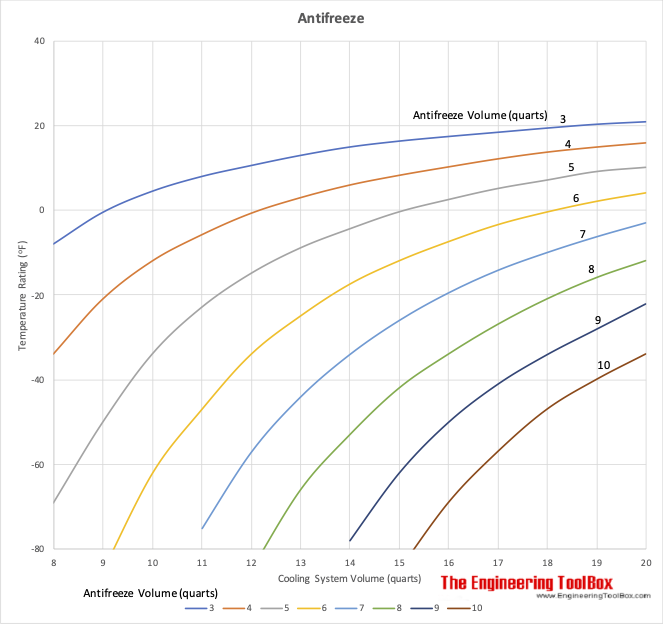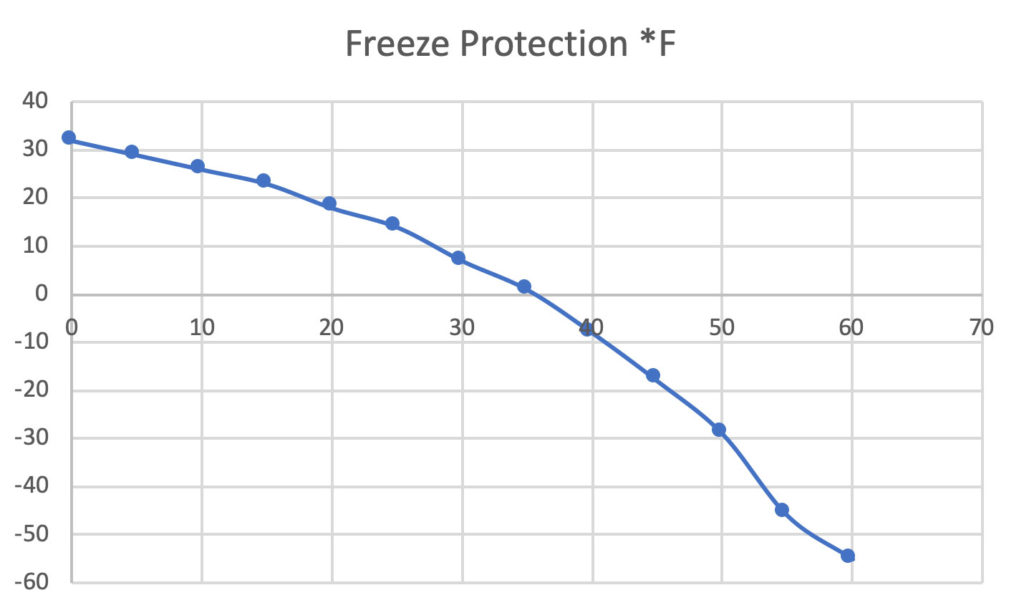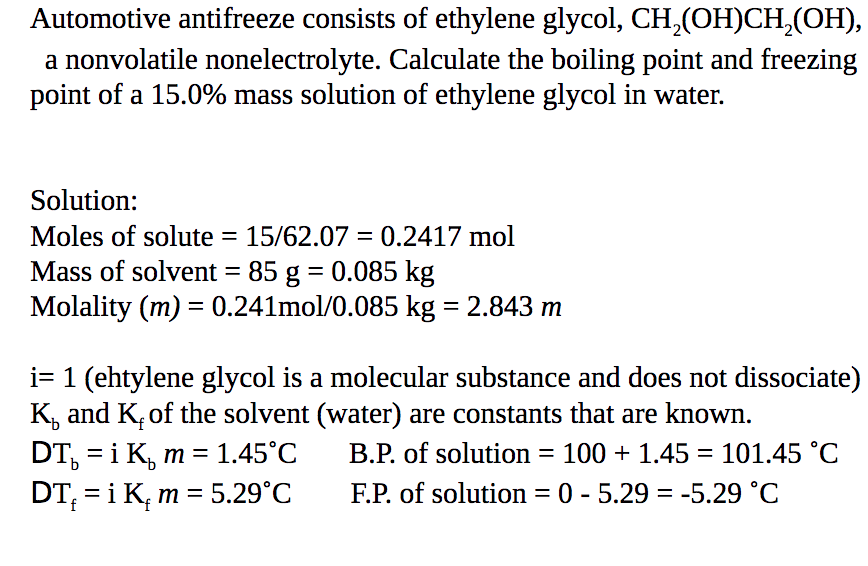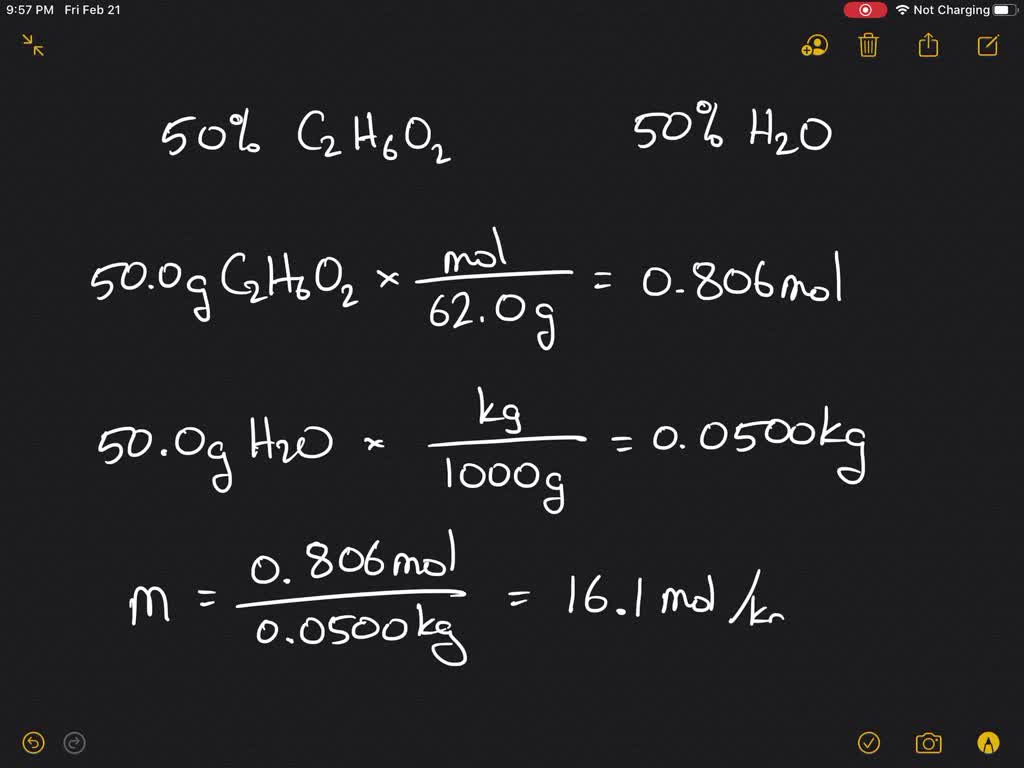
SOLVED:Calculate the freezing point and boiling point of an antifreeze solution that is 50.0 % by mass of ethylene glycol (HOCH2 CH2 OH) in water. Ethylene glycol is a nonelectrolyte.

An antifreeze solution is prepared from 222.6 g of ethylene glycol (C2H6O2) and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g

An antifreeze solution is prepared from 222.6g of ethylene glycol (C2H6O2) and 200g of water. - YouTube

SOLVED: An aqueous antifreeze solution is 33.0% ethylene glycol (CzH6O2) by mass The density of the solution is 1.042 g/cm?. Calculate the molality of the ethylene glycol m Submit Answer Tries 0/5
![An antifreeze solution is prepared from `222.6 g` of ethylene glycol `[C_(2)H_(4)(OH)_(2)]` and ... - YouTube An antifreeze solution is prepared from `222.6 g` of ethylene glycol `[C_(2)H_(4)(OH)_(2)]` and ... - YouTube](https://i.ytimg.com/vi/qDRYyKBs2_U/maxresdefault.jpg)