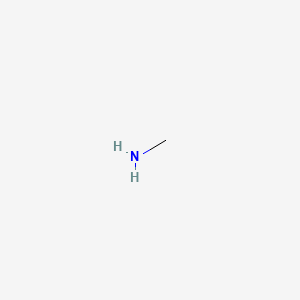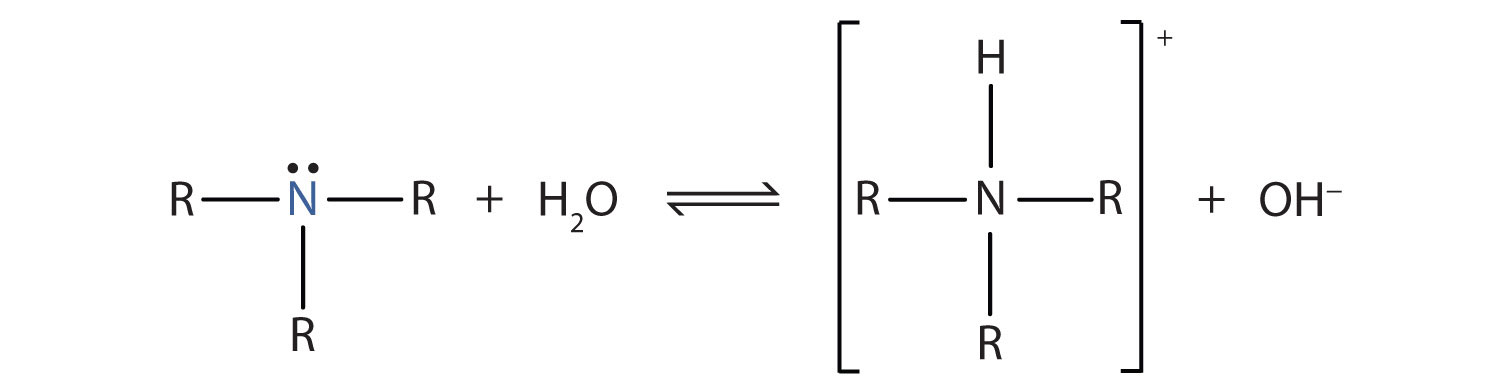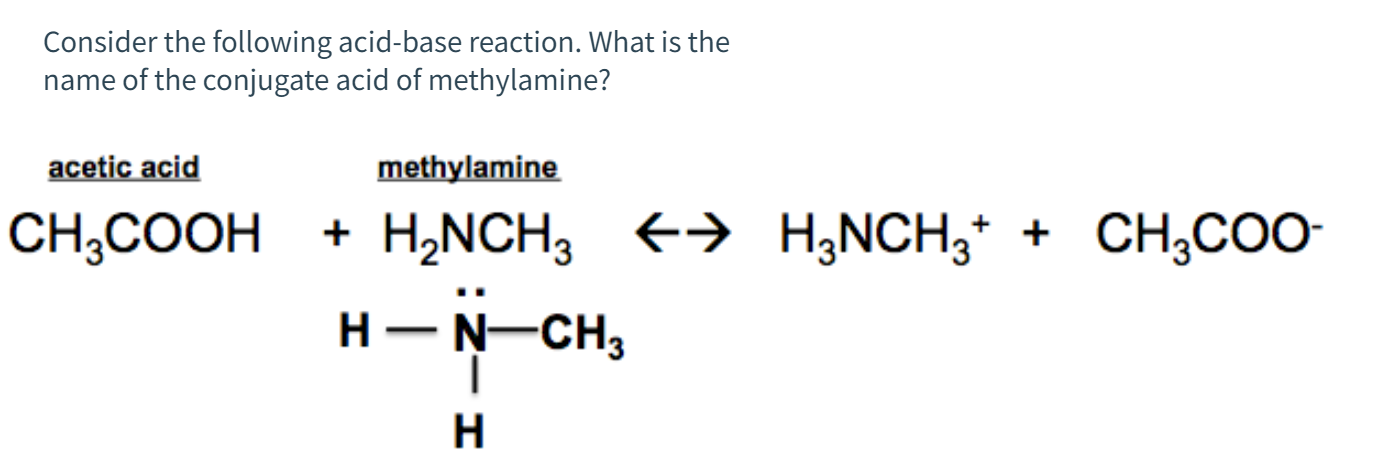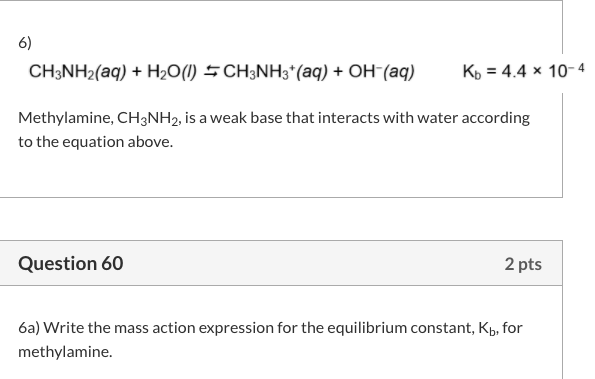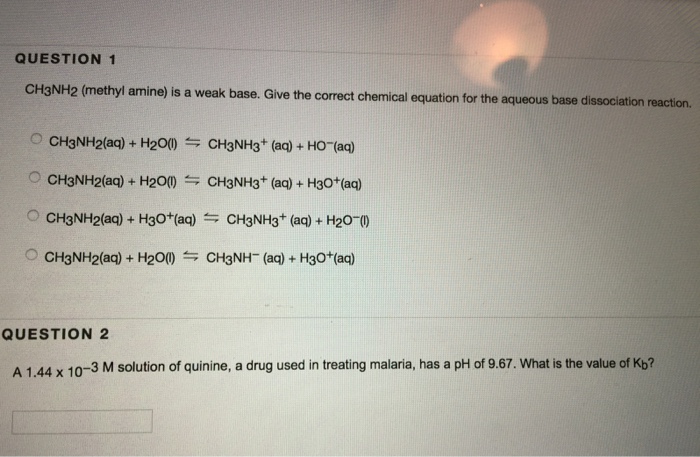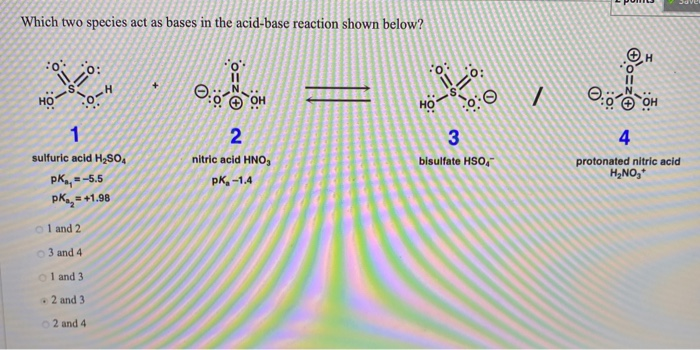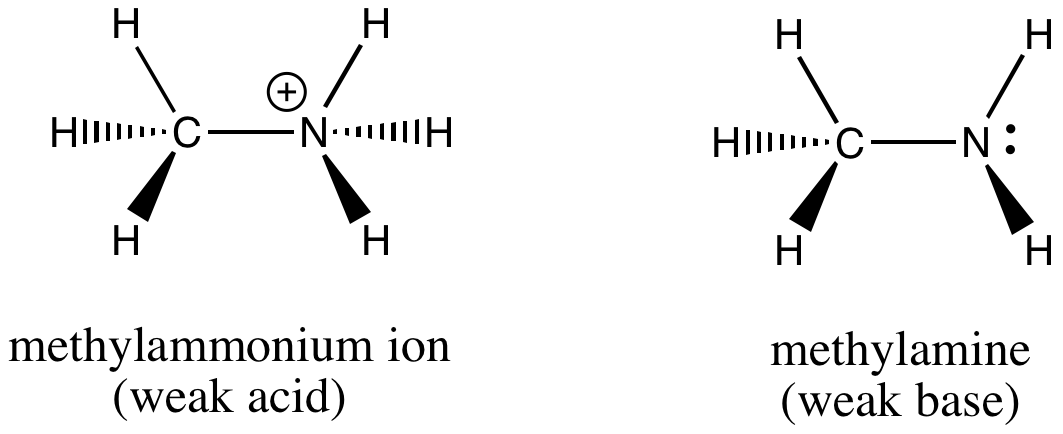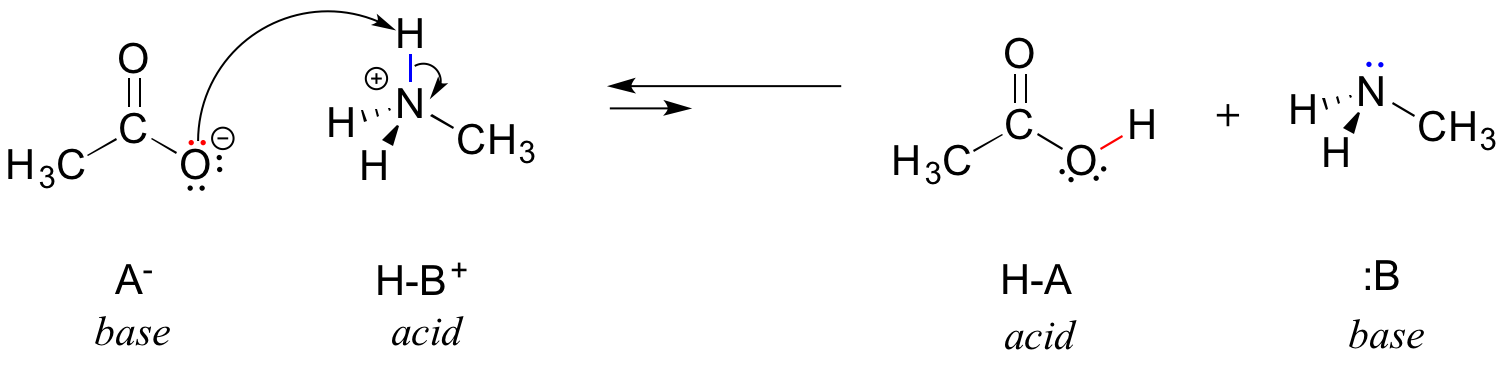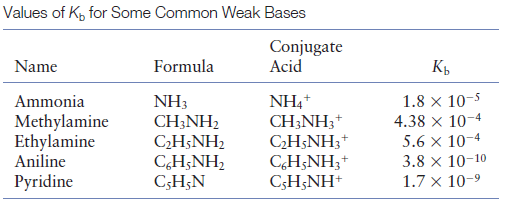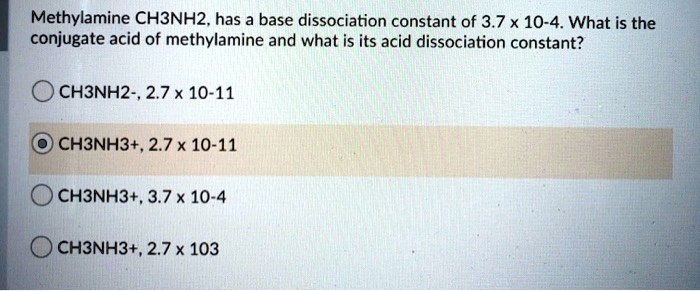
SOLVED: Methylamine CH3NH2, has a base dissociation constant of 3.7 x 10-4. What is the conjugate acid of methylamine and what is its acid dissociation constant? CH3NHZ -, 2.7 x 10-11 CH3NH3+,2.7
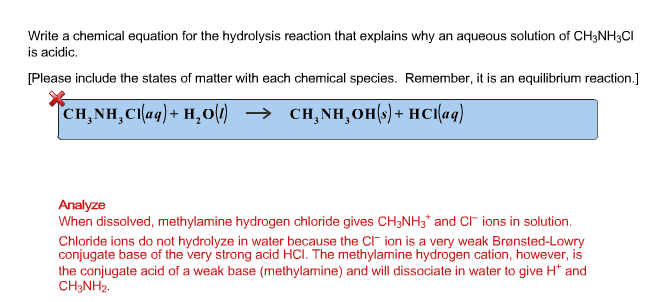
OneClass: Write a chemical equation for the hydrolysis reaction that explains why an aqueous solution...
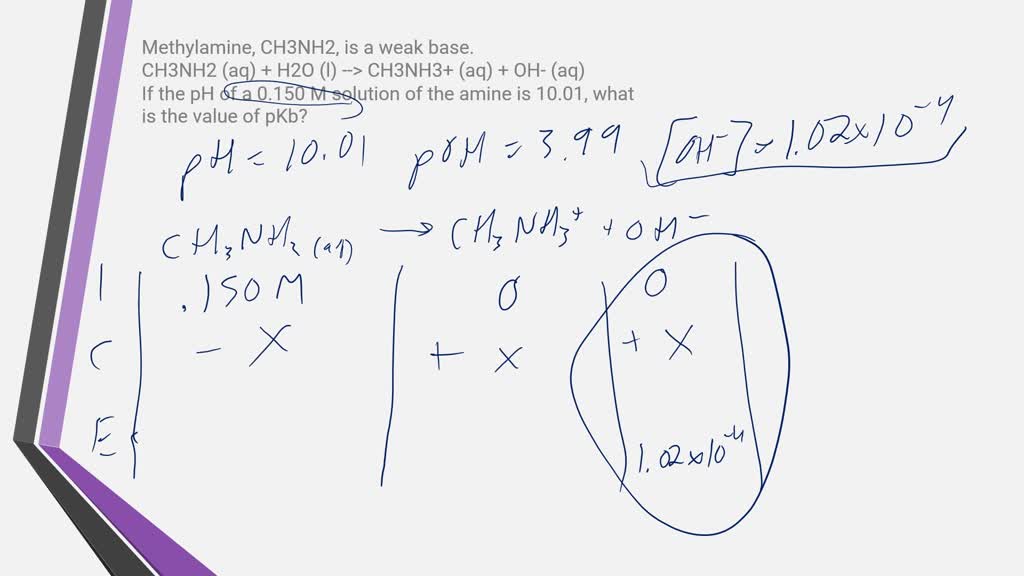
SOLVED: Methylamine, CH3NH2, is a weak base. CH3NH2 (aq) + H2O (l) –> CH3NH3+ (aq) + OH- (aq) If the pH of a 0.150 M solution of the amine is 10.01, what
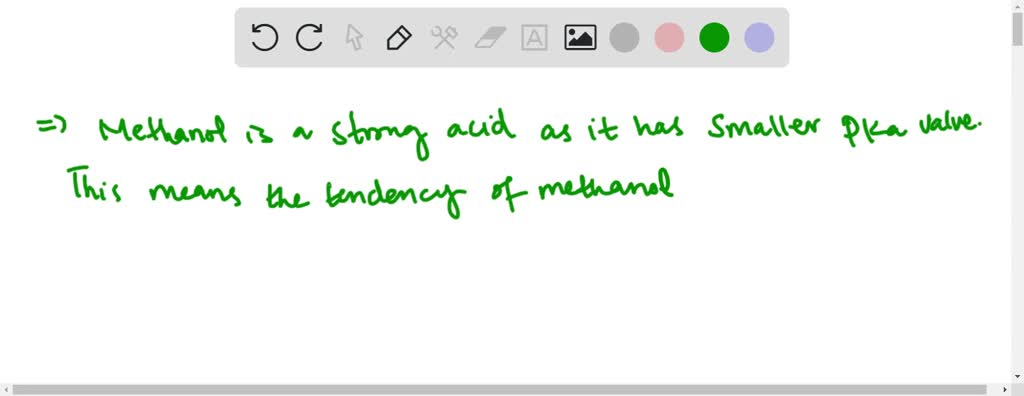
SOLVED:Does methanol behave as an acid or a base when it reacts with methylamine? (Hint: See page 55 for the structures of methanol and methylamine.)