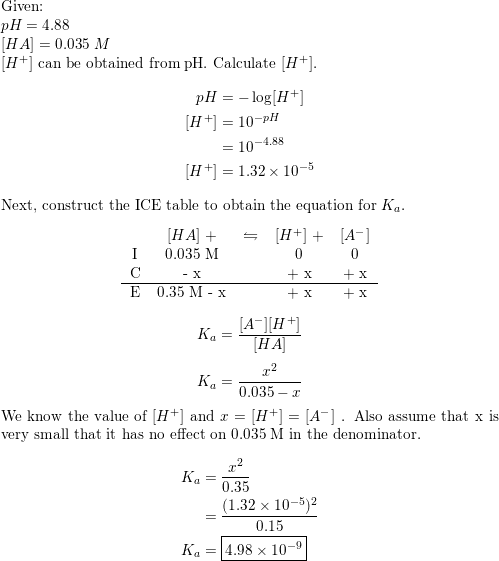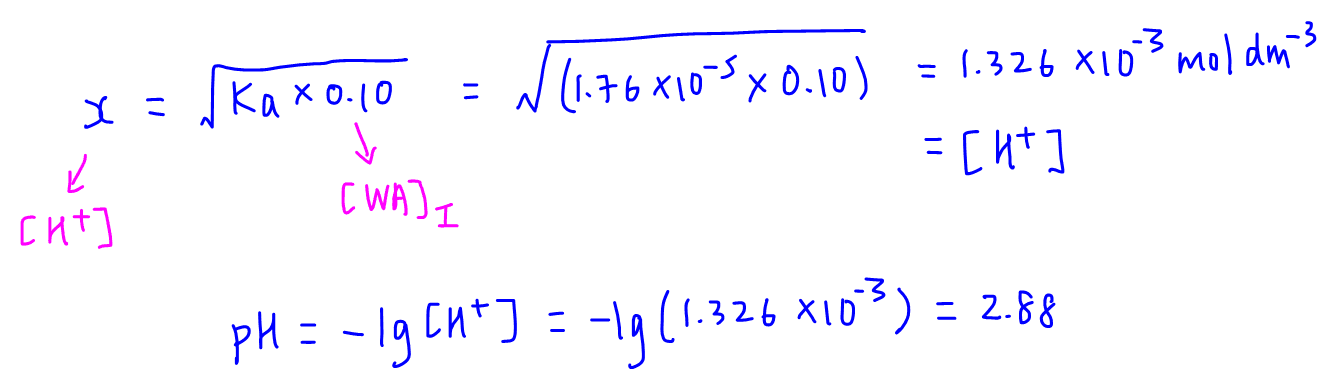
Calculation of Hydrolysis Constant, Degree of Hydrolysis and pH of Salt Solution - Chemistry, Class 11, Ionic Equilibrium
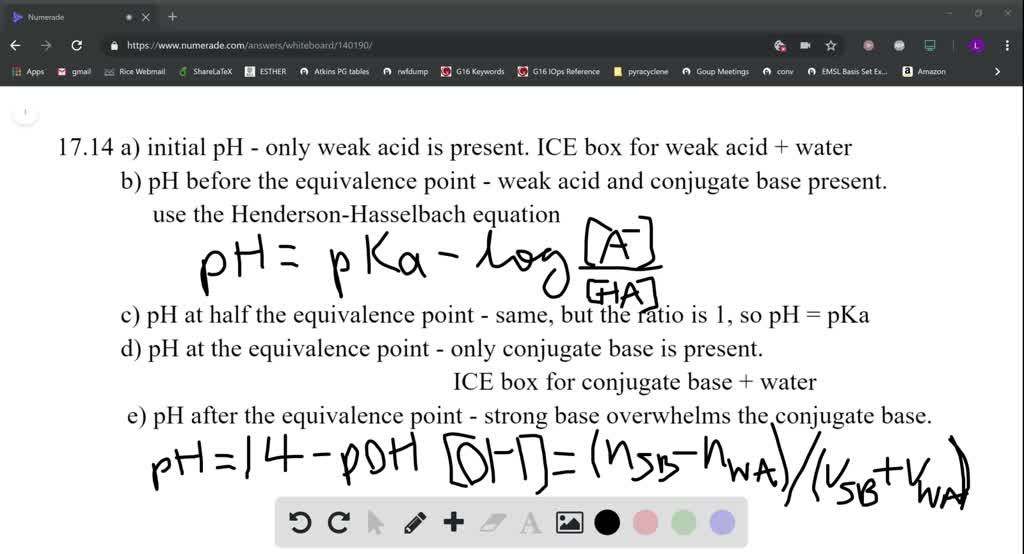
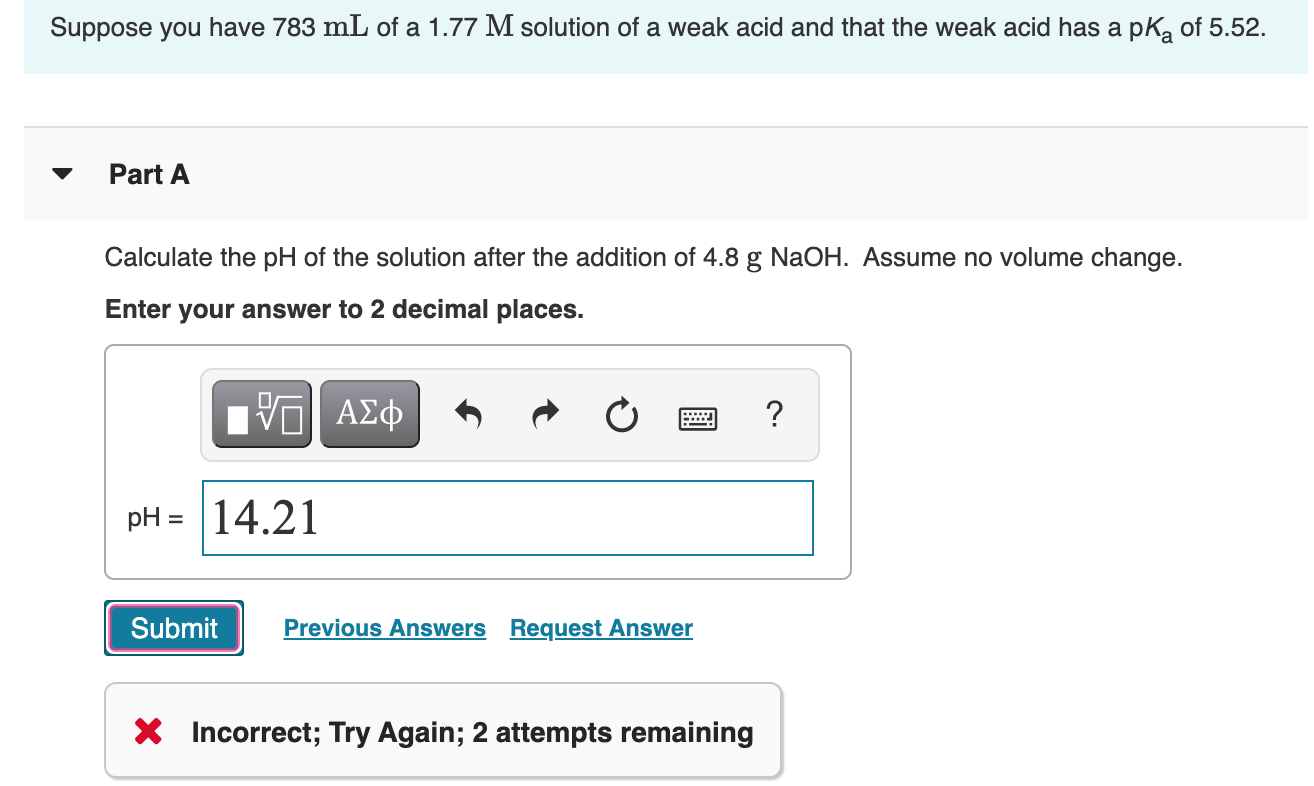
SOLVED:In the titration of a weak acid with a strong base, how do you calculate these quantities? a. initial pH b. pH before the equivalence point c. pH at one-half the equivalence

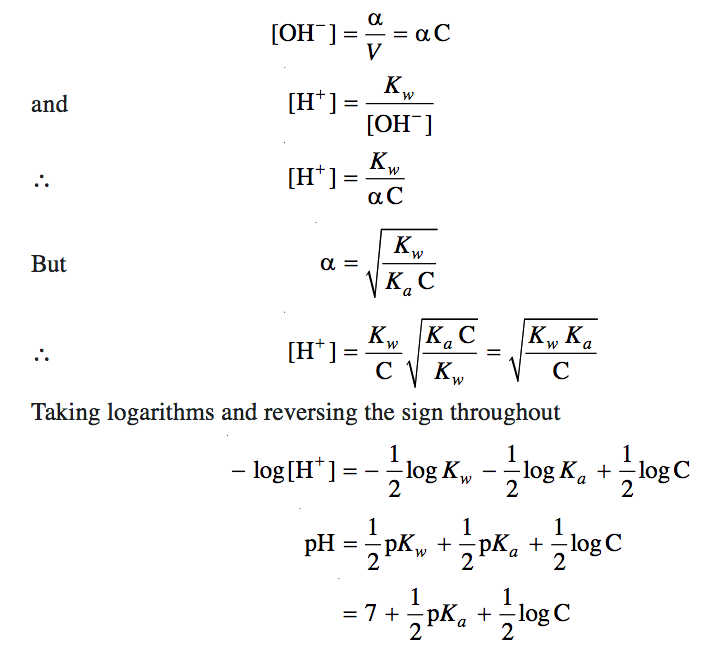
Calculate pH of a salt of weak monobasic acid and weak monoacidic base having concentration 0.1 M at 25^oC (Given : - pka = 4.8 pkb = 5.2 )